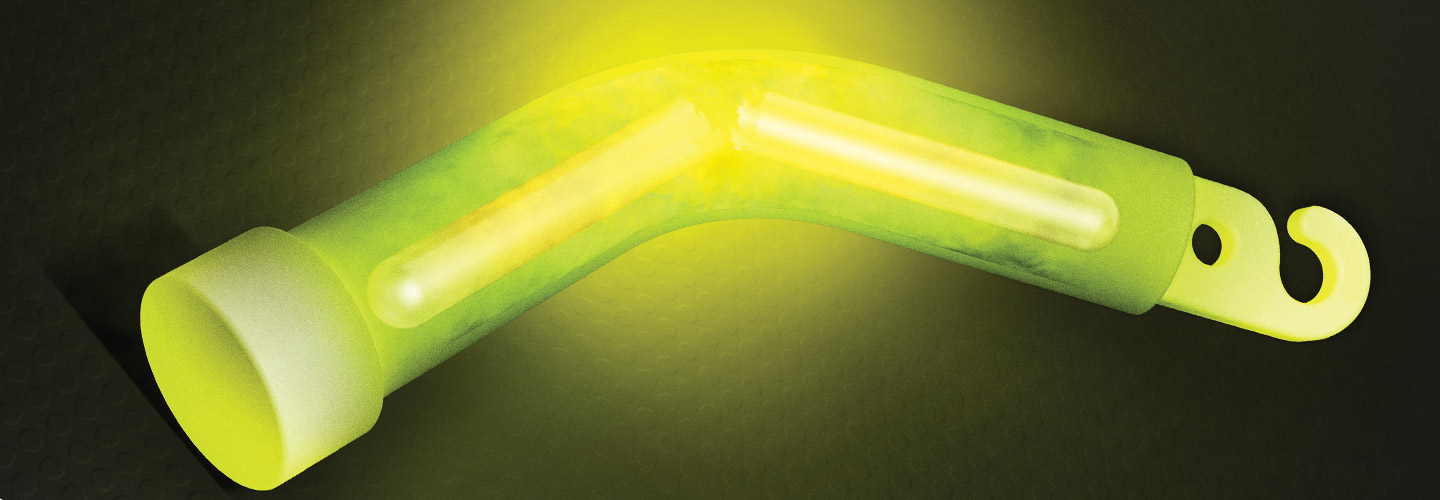
Trick-or-treaters often carry glow sticks to make themselves more visible in the dark as they travel door-to-door. But how exactly do these Halloween staples produce their eerie glow? They rely on chemical reactions, which occur when substances interact and change to create new substances. Some chemical reactions—like those happening inside glow sticks—give off light. This process is called chemiluminescence (ke-mee-loo-mih-NEH-sens).
Trick-or-treaters carry glow sticks as they travel from door to door. This makes them more visible in the dark. But how exactly do these sticks make their eerie glow? They use chemical reactions, which occur when substances mix and change to create new substances. Some chemical reactions—like those happening inside glow sticks—give off light. This process is called chemiluminescence (kem-mee-loo-mih-NEH-sens).